

Exercises with Radiation Dosage and Radiation Protection
1:
- It is in total 3.58 mole = 2.1562•1024 atoms of potassium in an average human, 12% of this is 2.5876•1020 is 40K
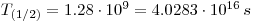
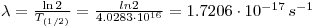
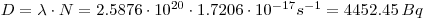
- First calculate the dose per second:
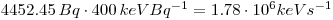
Then calculate it to joule per second:
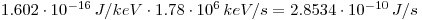
which gives:
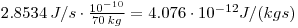
As the dose can be assumed to be constant throughout a human life, each year we will receive a dose of:
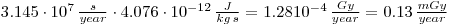
2:
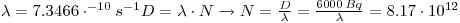
which is 1.36•10-11mole of 137Cs
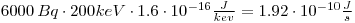
Calculate the initial dose rate from the reindeer meat:The dose of a person who weighs 70 kg equals to 2.74•10-12Gy/s.
Then calculate the real half-life
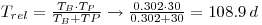
This gives:
![D_{tot}=\int^{t=\infty}_{t=0}D_{0}\cdot e^{-\lambda t}dt=-\frac{D_{0}}{\lambda}\left[e^{-\lambda t}\right]^{t=\infty}_{t=0}=\frac{D_{0}}{\lambda}(0-1)=\frac{2.74\cdot 10^{-12}}{7.367\cdot 10^{-8}}=0.580\, mGy/year D_{tot}=\int^{t=\infty}_{t=0}D_{0}\cdot e^{-\lambda t}dt=-\frac{D_{0}}{\lambda}\left[e^{-\lambda t}\right]^{t=\infty}_{t=0}=\frac{D_{0}}{\lambda}(0-1)=\frac{2.74\cdot 10^{-12}}{7.367\cdot 10^{-8}}=0.580\, mGy/year](math/0b495174d5fe8f347f8673dba4ea6818.gif)
3:
ALI (Annual Limit of Intake) is the amount of activity in a certain material that, when consumed, gives a radiation dose of 20 mSv. There is ALI-values for both inhalation and consummation of radioactive material.
Both chemical and physical properties can make a substance dangerous. Decay type, half-life and the energy of the radiation determines the dose received. The chemical form and the chemical properties of the material determine if it is accepted or if it gets enriched in the body. For instance an alpha-emitter can be inhaled and do a substantial amount of damage in the body. The combination of a short physical half-life together with a long biological half-life will deposit the greatest amount of dose in the body. Some examples of interest are iodine, which accumulates in the thyroid gland, polonium which accumulates in the bones and radon gas which can be accumulated in the lungs.
| Page | Date Edited |
|---|---|
| Calculation Exercises | Oct 14, 2015 |
| Suggested Solutions to Radiation Dosage and Radiation Protection | Mar 14, 2014 |