Lab Exercise - LSC Principles
Table 1: Typical radionuclides for LSC counting
Quantitative measurements of beta-radiation, alpha-radiation and low-energy gamma-radiation with traditional gas or solid state detectors may be difficult due to the self-absorption of the radiation in the sample itself and further absorption in the counter window. Therefore, low-energy radiation is, in many cases, best detected and measured with liquid scintillation counting where absorption loss is absent. Here, the sample and the detector (the liquid scintillator) is intimately mixed in a homogeneous solution. In addition, this leads to nearly 100% counting geometry (only the liquid surfaces against the container wall and the air above the liquid account for a small loss in counting geometry).
A liquid scintillator consists of a solvent and a primary scintillant. In some cases a secondary scintillant is also added. The function of these substances is described below.
Solvent
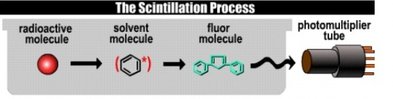
The primary function of the solvent is to dissolve the radioactive sample, preferentially into a homogeneous solution. An equally important function is to absorb and transfer the energy deposited by the radioactive decay event to the primary scintillate. The process is as follows: The β-particles from the radioactive substance ionize and excite molecules in the solution, - first and foremost the solvent molecules because of its high concentration. The excitation energy is transferred from one solvent molecule X to another. Some of these excitation transfer chain reactions result in the encounter with a molecule of the primary scintillant Y. The excitation is transferred to this molecule:
β + X1 → X1* + X2 → X2* + X3 → X3* ... → Xn* + Y → Y*
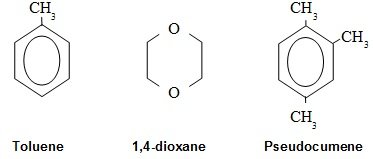
Figure 1: The most common Liquid Scintillation Solvents
Commonly used solvents are toluene and 1,4-dioxane. The first is used for organic solutions and the latter when the radioactivity is dissolved in an aqueous solution. Many modern scintillation cocktails are based on the solvent pseudocumene because of lower vapor pressure and higher flash point than the two first. Modern cocktails also contain surfactants of different kinds and in varying degree in order to facilitate solubilization of a large variety of solutions, both aqueous and organic.
The Primary Scintillator
A scintillant exhibit properties which in many respects are just the opposite of those of solvents. They absorb the energy of the solvent molecule and tend to decay rapidly mainly through the emission of light photons, thus having a high quantum fluorescent yield. The wavelength of the light emitted should be compatible with the sensitive wavelength region of the photo-cathode in the PM-tube. The concentration of the scintillant is in the area 3-5 g/L, i.e. a relatively dilute solution. Accordingly, a useful scintillant should have the following properties:
Accordingly, a useful scintillant should have the following properties:
- High absorption of energy from solvent molecules.
- Emission of light matching spectral sensitivity of the photo cathode.
- Solubility in solvent.
- Adequate chemical stability and stability against radiation degradation.
- Short fluorescence time.
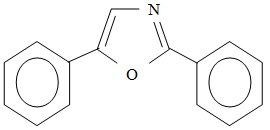
A common primary scintillant is 2,5-diphenyloxazole, also called PPO. See Fig 2
Figure 2: Structure formula of the most common primary Scintillate PPO
Figure 3: Basic sketch of the primary scintillation process
The Secondary Scintillant
Early scintillation counters were sometimes unable to detect the short wavelengths emitted by primary scintillants; as a result, secondary scintillants Z were added to amplify the primary emissions. Secondary scintillants are also complex organic compounds with the ability to absorb the decay energy of the primary scintillant and rapidly emitting it at a longer wavelength, shifting the overall signal to a wavelength more easily detectable by photomultiplier tubes:
Y* → hv1 + Z → Z* → hv2
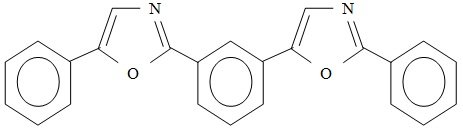
Figure 4: Structure formula of the most common secondary scintillant POPOP
As more sensitive photomultiplier tubes were constructed, secondary solutes became in principle unnecessary. However, they may still be used to improve counting efficiency, as both the shorter and longer wavelengths can be detected.
A common secondary scintillant is 1,4-di(2-(5-phenyloxazyl))-benzene, also called POPOP. The secondary scintillant is also called the wavelength shifter. Its concentration is in the range of 0.1 g/L.
β energy | ||||||
Half-Life | Decay | Emax | Emin | Monitor | Specific Activity | |
125I | 60 days | γ | Auger electrons | 0.035 MeV | γ-probe | 2167 Ci/mA |
3H | 12.3 years | β | 0.019 MeV | 0.035 MeV | Swabs | 28.8 Ci/mA |
14C | 5730 years | β | 0.156 MeV | 0.006 MeV | β Counter | 62.4 Ci/mA |
25S | 87.4 days | β | 0.167 MeV | 0.049 MeV | β Counter | 1494 Ci/mA |
32P | 14.3 days | β | 1.709 MeV | 0.69 MeV | β Counter | 9311 Ci/mA |