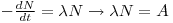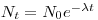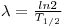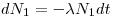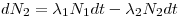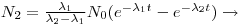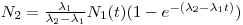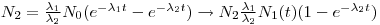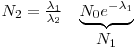Radioactive disintegration is a stochastic process, which means a random process that can be described statistically. Here you will learn about the secular radioactive equilibrium, and how any measure of a radioactive source is stated with uncertainty.
The Basics - A Single Radioactive Nucleus
In a sample with N radioactive atoms of a particular nuclide, the number of nuclei that disintegrates with the time
dt will be proportional with N, see eq. 1:
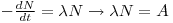
|
eq. 1
|
where λ is the disintegration constant and A is the rate of disintegration.
Eq. 1 is a simple differential equation and can be solved using standard mathematical techniques. The solution is written:
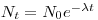
|
eq. 2
|
N
0 is the number of nuclei at present at t = 0. The time when half of the nuclei has disintegrated is called the half-life.
At t = T1/2 then N = N
0/2. If we insert this into eq. 2 the following connection between the disintegration constant and the half-life is obtained:
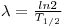
|
eq. 3
|
The half-life is a characteristic value for each radioactive nuclei.
Decay Chains and Mother-Daughter Relationships
A radioactive nuclide will often disintegrate into a product that is radioactive as well: Nucleus 1→Nucleus 2 →Nucleus 3. The initial nucleus is usually referred to as the mother nuclide and the product as the daughter nuclide.
Assume that at the time
t = 0, N
0 of the mother is
N1(t =0), N2(t=0) and N3(t=0), the change in number of mother- and daughter nuclei can then respectively be described through Eqn 4 and Eqn 5:
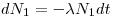
|
eq. 4
|
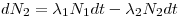
|
eq. 5
|
The solution of Eqn 4 is already known, it is the expression in eq. 2 while the solution for the amount of daughter nuclei are given with:
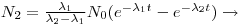
|
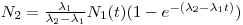
|
Eqn 6
|
If the half-life of the mother is much less than that of the daughter, Eqn 6 can be simplified into:
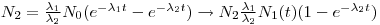
|
Eqn 7
|
where 1-e
λ(2)t is the saturation factor and λ
2-λ
1~λ
2. The above equation can be further reduced by the assumption that t >> T
½(2) (the observed time is much larger than the daughters half-life).

|
Eqn 8
|
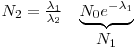
|
Eqn 9
|
When e
λ(2)t→0, eqn 9 is called a secular radioactive equilibrium and can be written as λ
2N
2 = λ
1N
1.