The median composition of the Askola groundwater is given in table 1:
Table 1. Median composition of the Askola ground water (GTK 2008)
| mg/L |
|
| HCO3- |
128 |
| SIO2 |
11.7 |
| Na+ |
34.05 |
| K+ |
2.36 |
| Ca2+ |
14.05 |
| Mg2+ |
6.33 |
| Sr2+ |
0.13 |
| B3+ |
0.05 |
| SO42- |
17.85 |
| Cl- |
25.6 |
| F- |
0.38 |
| Br- |
0.02 |
| I- |
0.02 |
The pH of the water is 7.7, temperature 10 centigrade and the uranium concentration 340 μg/l. In oxic conditions the Eh of the water is 500 mV and the density of water 1.0 kg/l.
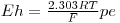
where R is the gas constant, T temperature and F the Faraday constant
R = 8.3145 J K-1 mol-1 F = 96485.3 C/mol
Modify the given .phrg input file (below) based on data given above and do the following exercises using PHREEQC:
1. Calculate the prevailing uranium species at pH range 2-12 using llnl.dat database. Create a "selected output" file with most relevant prevailing uranium species. Open your .txt file with excel or another relevant program and produce a figure of uranium species as a function of pH. What uranium species are most prevailing in oxic conditions? Save your output file as a text file for the next exercise.
2. Open your output file, and from ”Saturation indices” block look at the SI-values. Write in your input file a command line for the SI values of the most relevant solid uranium phases and produce a figure representing the SI values as a function of pH.
3. If we assume that a uranium-bearing groundwater attains equilibrium with a strongly reducing mineral, such as FeS, how will pH, pe, uranium species and SI values of solid uranium phases change?
Input file for modification based on given data:
SOLUTION 1 Speciation exercise #use llnl-database
#write the correct values, calculate pe based on Eh:
-temp
-pH
-pe
-units
- write the water composition here:
SAVE SOLUTION 1
END
USE SOLUTION 1
#SELECTED_OUTPUT
#-RESET false
#-file #here the name of the file
#-PH
#-TOTALS #here the oxidation states of uranium
#-molalities #here the species of interest
#-SATURATION_INDICES #here satiration indices
PHASES
Fix_H+
H+ = H+
log_k 0.0
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -2.0 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -3 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -4 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -5 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -6 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -7 HCl 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -8 NaOH 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -9 NaOH 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -10 NaOH 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -11 NaOH 10.0
END
USE solution 1
EQUILIBRIUM_PHASES 1
Fix_H+ -12 NaOH 10.0
END