- Great care should be taken to avoid getting uranium compounds in solid or liquid form on the skin as uranium is extremely toxic as well as radioactive.
- A lab coat, disposable gloves and safety glasses should be worn at all times.
- DO NOT dispose of any liquid or solid waste down sinks or in waste bins. Place in the red waste buckets provided.
Aim
To separate 234mPa from uranyl nitrate and to measure its half-life.
Theory
One of the four natural radioactive decay series is the (4n + 2) series which starts at 238U. The first few steps in the series may be represented as follows:
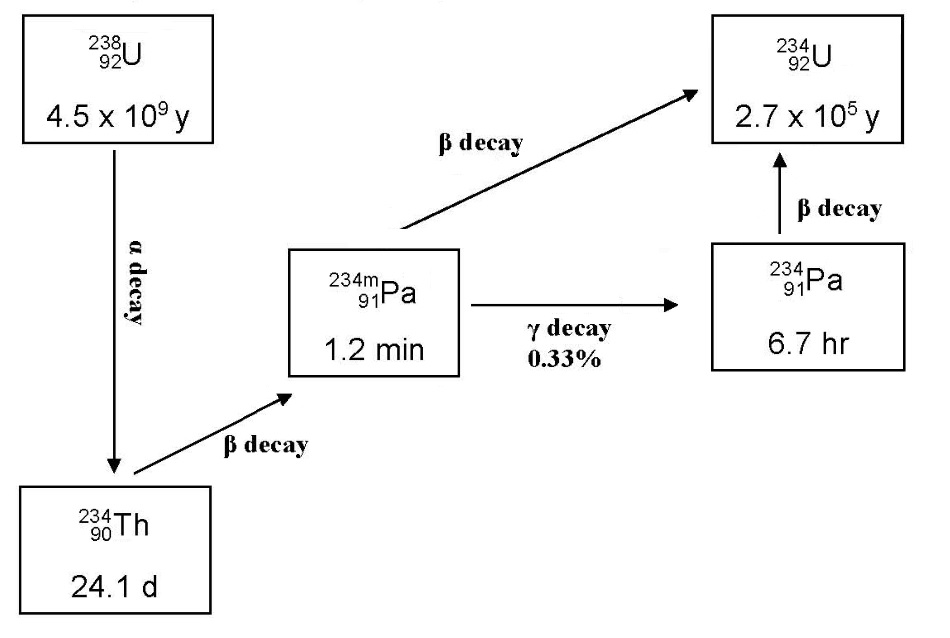
In any sample of natural uranium, although the bulk may be 238U, small amounts of 234Th, 234mPa, 234Pa and 234U will be present. Since thorium and protactinium are chemically different from uranium, chemical separation methods may be used to isolate these elements from a sample of natural uranium. This experiment, involves the separation of 234mPa (and 234Pa, although the amount of this will be too small to measure) by the use of an ion exchange resin.
You are provided with an ion exchange column containing Amberlite IR-120, a sulphonated polystyrene cation exchange resin. If a solution of 238U, 234Th and 234mPa is poured through this resin at approximately pH 7, all three metal ions exchange with H+ ions on the column and are absorbed onto the resin. However, at lower pH values H+ ions can successfully displace 238U (which in this experiment is in the form of the uranyl ion UO22+) and the 234mPa, leaving only 234Th adsorbed. If 234Th is left alone on the column for a few minutes its radioactive decay gives rise to 234mPa, which can then be removed from the resin by passing more acid through the column.
Experimental procedure for the separation of 234mPa from 238U and measurement of its half-life
Questions - Sepataion of 234mPA from 238U and measurment of its half life