The yield of nuclear reactions can be found through the formula below:
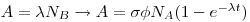
where:
- A is the activity of the produced nucleus B (which you calculate from your efficiency curve)
- λ is the half life of the product nucleus B
- NA is the number of atoms of the target nucleus (in the case of exercise 3 this is the amount of the unknown metal)
- σ is the cross section (the likelihood of a reaction between the neutrons and the target nucleus A)
- φ is the neutron flux (the number of neutrons hitting the target)
What this equation tells you is that if you irradiate NA atoms of A (usually referred to as your "target") with neutrons of intensity φ for a time t, the disintegration rate of your product will be A (do not mix the target nucleus A with the activity, A, of the product nucleus B!).
You'll find sigma in the Chart of the Nuclides (it's around 20 for the unknown metal). Its unit is in barn, which is equal to 10-24 cm2. The neutron flux is given in the exercise text and is equal to 105 n1s-1cm2 . When you multiply these two numbers you are left with s-1, which is the correct unit for the disintegration rate.