Developed by
Learning Goals
Explanation and Exercise Guide
Theory
In the extraction of an inorganic substance by an organic solvent the former must be present in a chemical form that is soluble in water and the organic solvent used. Extraction systems for metal ions are generally based on the formation of coordinated complexes or ion association complexes. Complexes can be formed with simple ligands as shown below (taken from ‘Solvent Extraction in Analytical Chemistry’ Morrison & Freiser).
NH3 > RNH2 > R2NH > R3N
H2O > ROH > R2O > R2CO3 > RCHO
R3 > R3P > R2S
Where R = alkyl or aryl group
The relative strengths of the complexes formed with simple ligands approximately correlates with the relative strengths of the ligands as Lewis bases. The formation of a complex with simple ligands does not necessarily confer solubility in an organic solvent. The solubility must be conferred by the formation of a soluble association complex with the organic solvent used. Chelate compounds formed with polyfunctional ligands such as dimethyl glyoxime are often used for solvent extraction. Heteropoly acids such as silicomolybdic can also be used. In some cases the covalent nature of the substance itself may allow extraction into a non-polar solvent (i.e. the halogens).
The % of substance extracted and the distribution ratio (D) are related by the equation:
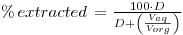
The value of D in any system must be determined empirically for a variety of conditions. For a given system D will depend on:-
- Ratio of volumes Vaq: Vorg
- Temperature (normally D varies inversely with temperature)
- Concentration of organic solvent when a diluent is used
- Concentration of other reagents i.e a chelating agent in the organic solvent, HCl in the water phase, etc
- The presence of interfering substances which may
- Form non-extractable compounds with the substance required
- Change the ionic strength of the aqueous phase
- Itself extract altering the properties of the extracting solvent i.e. HNO3 in the extraction of uranyl nitrate
The PUREX process uses solvent extraction to separate uranium and plutonium. To show this principle at work, this experiment will extract phosphorous from an aqueous solution by converting it into phosphomolybdic acid and using ethyl acetate to extract it.
Experimental Procedure
Experimental procedure for the separation of radioisotopes by solvent extractionQuestions for the Students
Other
How to do calculations or other important aspects for the theory that is not directly related to exercise.Safety Aspects
- All work with 32P must be carried out in the spill tray provided, and a lab coat, gloves and safety spectacles must be worn. Heating of solutions should be carried out using a dry bath housed in the fume-cupboard.
- Planchetted samples should be carried to the Geiger counter on a tray and should only be handled with tweezers.
- DO NOT dispose of any liquid or solid waste down sinks or in waste bins. Place waste liquids in the plastic waste beakers provided. Place used gloves and tissues in the bin bags at the ends of the benches.
Equipment
Description of the equipment needed and used during the exercises.Preparation for the lab Supervisor
The preparation that the lab Supervisor needs to do to ready the lab.Feedback from Users and Supervisors
Leave feedback in comments
