Return to Exercises with the Chart of the Nuclides

These answers are based on the Karlsruher Nuklidekarte.
1:
The color gives information about the type of disintegration. Red = β+/e, blue = β-, yellow = α, orange = proton, green = fission.
2:
- Isotopes are nuclides with an equal number of protons, but with a different number of neutrons. They are aligned on a horizontal line in the Chart of the Nuclides, e.g. 28Si and 29Si.
- Isotones are nuclides with the same number of neutrons but different number of protons. They can be found along a vertical line in the Chart of the Nuclides, e.g.28Si and 29P.
- Isobars are nuclides of different elements that have the same number of nucleons and can be found along a diagonal line from the left top corner to the bottom right corner. e.g.28Si and 28P.
3:
Uranium, with the isotopes 234U,235U and238U, is the heaviest naturally occurring element. Half of the square is black and the other half indicates what kind of radiation that is emitted. The above mentioned color marking means that the nuclide exists natural, but is radioactive and slowly disintegrates towards stability. 238U has approximately ten times longer half- life than 235U and this is the reason why there is much more 238U than 235U (the amount was similar during the formation of the earth.234U exists as a daughter product in the 238U series.
4:
Bismuth was long thought to be the heaviest stable elements with the nuclide 209Bi. α-decay from this nuclide has been observed and therefore 208Pb is the heaviest stable nuclide [1] .
5:
- 40Ar is the most abundant isotope of Ar, 99.6%. per atom
- 39Ar has the longest half-life, which is 269 years.
- 35Ar, 45Ar,46Ar, and 47Ar
- 43Ar emits γ-quants, 975 keV, 738 keV and 1440 keV, when disintegrating. These values can be read in the Chart of the Nuclides and they are the most common energy levels (highest intensity), in reality there exists numerous more which can be found in various tables. The gamma rays are arranged from decreasing intensity. In this example 975 keV has the highest probability of being emitted .
- The square to the stable nuclides contains information about chemical symbol, isotope number, abundance and cross section absorbance for thermal neutrons, which means the probability of a nuclide to absorb a thermal neutron and form the isotope with one more neutron.
- The color of the square indicates the type of radiation it can emit. The energy of the radiation is given after the symbols (β+/-) in MeV.
- An eventual γ-radiation is noted under the particle radiation, arranged in decreasing intensity with keV as energy units.
32Si has the longest half-life, 172 years and disintegrates according to the following:
32Si⟶32P⟶32S
8:
There are three shielded Pm isotopes, namely 144Pm,146Pm and 148Pm. The Pm isotope with the longest half-life is 145Pm (17.7 years), 146Pm (5.53 years) and 147Pm(2.62 years). They disintegrate according to the following:
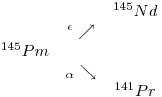
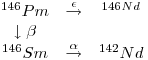
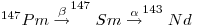
9:
β- disintegration occurs mainly on the neutron rich side in the Chart of Nuclides and β+ on the neutron poor side. A nucleus that can emit both positrons and negatrons usually have odd-odd configuration of protons and neutrons. In addition it has to lie close to the valley of stability.
10:
See the Chart of the Nuclides. Notice it can disintegrate with α, β+and βp, in addition some of the daughters have several ways to disintegrate.
11:
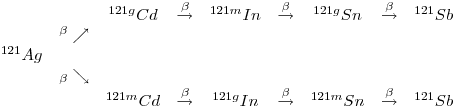
12
A nucleus can reach stability in several different ways, by different kinds of radiation. When a square is divided in two equal parts, as with 211Ra, each part stands for between 5% and 95 % of the disintegration. When one of the colors only covers a small size of the square, like with 214Ra, it means the radiation stands for less than 5% of the disintegration.
13:
See the Chart of the Nuclides.
14:
From the answer to question 13 it can be seen that 232Th disintegrates through a long chain of daughter products, where 232Th has no high intensity y lines. Not until the disintegration of the 3rd daughter, 224Ac, can any high intensity γ-lines be seen. In a newly prepared salt, none of the daughters will have had time to be formed in detectable amounts, and no γ-radiation can be seen. Though over time, equilibrium between Th and the daughters will be established. Several of the daughters have strong y and high energetic γlines, for instance 228Ac (911, 969…keV).
15:
Due to energetic reasons β+decay can only happen if the mass of the mother nucleus is at least two electron masses larger than that of the daughter. If the difference in mass is less than the above-mentioned electron capture becomes a competing alternative disintegration mechanism. 201Tl disintegrates only with electron capture; 197Tl has less than 1 % β+. In both situations
γ is also emitted.