

Exercises with Amount of Radioactive Material (number of nuclei, number of moles, weight) and the Law of Radioactive Decay
1:
- 3.2 • 108 Bq
- 383 Bq
- 2.3 • 109 Bq
- 8.0 • 104 Bq
2:
- 1.77 • 1013 atoms.
- 2.00 • 1011 atoms.
3:
2.40 g 60Co.
4:
- 2.53 • 105 Bq.
- 7.21 years = 7 years and 76.7 days.
5:
The mass of 99mTc is 20.5 ng. After one week 28 half-lives will have past, and the activity will be the following:
D = D0 • (½)28=4•07 Bq•(½)28=0.15 Bq.
Approximately all 99mTc has disintegrated and turned into 99Tc. The number of 99Tc atoms will therefore be the same as the original number of 99mTc atoms.
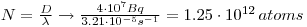
6:
- 1 g natural Lu contains 0.0259 g 176Lu, with a half-life equal to 3.8 • 1010 years, which gives A = 51 Bq
- There are two naturally occurring radioisotopes of Sm, 147Sm and 148Sm, which gives A = 127.24 Bq + 0.001 Bq = 127 Bq.
7:
3002 kg
8:
- 18.5 kg
- After 2.455 • 105 years it will be 2.299 • 108 Bq 238U left, which is equal to 18.49 kg.