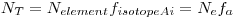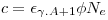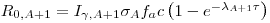Back to Main
We have:

|
(1)
|

|
(2)
|
Here
σ is the cross section of the reaction with thermal neutrons, φ is the flux of the neutrons, N
T is the total number of atoms in the sample,
I𝜸 is the relative intensity of the
𝜸-ray we are looking at, A
0 is the activity of the sample at time zero and R
0 is the count rate of the sample at time zero.
If the target contains several isotopes, as silver does, we must take into account the isotope fraction, for each isotope in our target:
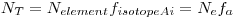
|
(3)
|
Where the right part of the equation shows the abbreviated indexes we are going to use.
E.g. for silver there are two stable isotopes:
107Ag and
109 Ag, with isotope fractions equal to 51.839% and 48.161% respectively. N
e equals the total number of silver atoms in your sample.
So, combining (1),(2) and (3) we end up with:

|
(4)
|
This formula will calculate the cont rate for isotope A+1 after an irradiation time
𝛕 of stable isotope A. I𝜸 =1.76% for the 633 keV 108Ag
𝜸-ray and 4.5% for the 658 keV
110Ag.
We can relate the count rate for the two silver-isotopes by observing that φ and N
e if course will be equal and that
ε𝜸,A+1
for the two 𝜸-rays (at 633 keV and 658 keV, respectively) will be practically equal since the energies are so close. I.e we define a constant:
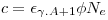
|
(5)
|
which do not depend on the irradiated isotope.Then:
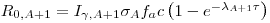
|
(6)
|
We can determine c from e.g the measurement of R0,110 from the 144 s irradiation of 110Ag:

|
(7)
|
Then theoretical values for R0,108 and R0,110 for any irradiation time can be estimated. In the laboratory exercise you will measure the R0 values for both isotopes for irradiation times 𝛕 =12,24,48,72 and 144 s. Thus, by using the value from (7) you can estimate the values for the nine (9) other cases and compare them to the measurement values.