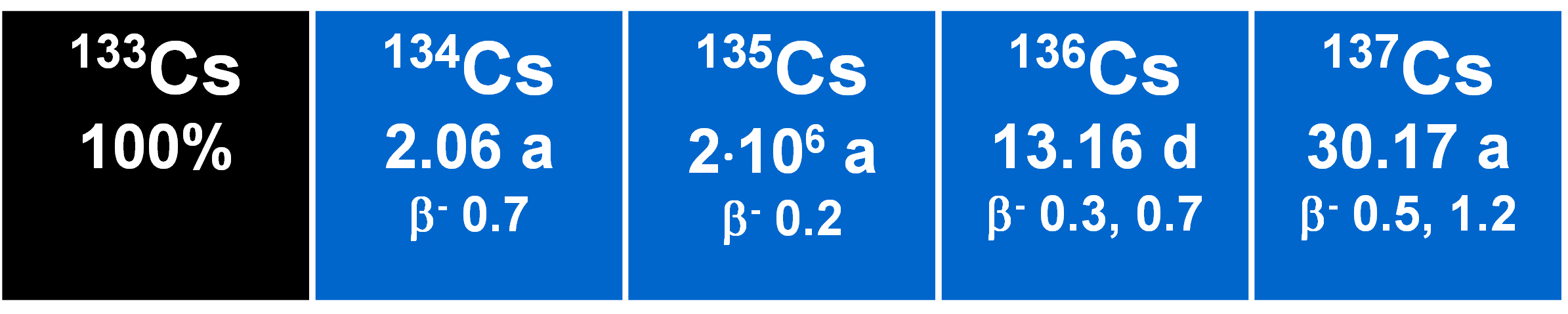
The natural isotope 133Cs is stable, but two radioactive isotopes of cesium are of interest. These are 135Cs and 137Cs. Both are radioactive with half-lives of 2.3 My and 30 y, respectively. They are produced with equal yields of about 6 to 7 % in binary fission of actinide nuclides. The nuclei are introduced into the environment through atmospheric nuclear explosions and release from the nuclear industries. 137Cs is a well-known radionuclide (and radiotracer) and time marker while 135Cs has been neglected because of its much longer half-life precludes detection by counting. In addition, in conventional mass spectrometers, the tail from the stable 133Cs swamps its signal (since the abundance of 135Cs is typically 10-9 smaller). The first measurement of the relevant cesium isotopes was made using two coastal sediment samples and a thermal ionization mass spectrometer. The [135Cs/133Cs] ratio was about 1•10-9 and the [137Cs/135Cs] ratio was about one to five due to the decay occurring through the past thirty years from their production ratio of about one. It was noted that this appeared to be the first detection of fallout 135Cs in nature and it may be inferred that the isotope ratio [137Cs/135Cs] is much more powerful as a tracer than 137Cs alone. Two models were given to illustrate how the ratio may be used in order to quantify estimates of recent sedimentation and rates of erosion (Lee et al. 1993).